 “Folding of
the Villin Headpiece Subdomain from Random Structures. Analysis of the Charge
Distribution as a Function of the pH.” D.R. Ripoll, J.A. Vila and H.A.
Scheraga. (2004). J. Mol. Biol. 339, 915-925.
[pdf]
“Folding of
the Villin Headpiece Subdomain from Random Structures. Analysis of the Charge
Distribution as a Function of the pH.” D.R. Ripoll, J.A. Vila and H.A.
Scheraga. (2004). J. Mol. Biol. 339, 915-925.
[pdf]This project is carried in collaboration with H. A. Scheraga and members of his group at the Department of Chemistry at Cornell.
 “Folding of
the Villin Headpiece Subdomain from Random Structures. Analysis of the Charge
Distribution as a Function of the pH.” D.R. Ripoll, J.A. Vila and H.A.
Scheraga. (2004). J. Mol. Biol. 339, 915-925.
[pdf]
“Folding of
the Villin Headpiece Subdomain from Random Structures. Analysis of the Charge
Distribution as a Function of the pH.” D.R. Ripoll, J.A. Vila and H.A.
Scheraga. (2004). J. Mol. Biol. 339, 915-925.
[pdf]
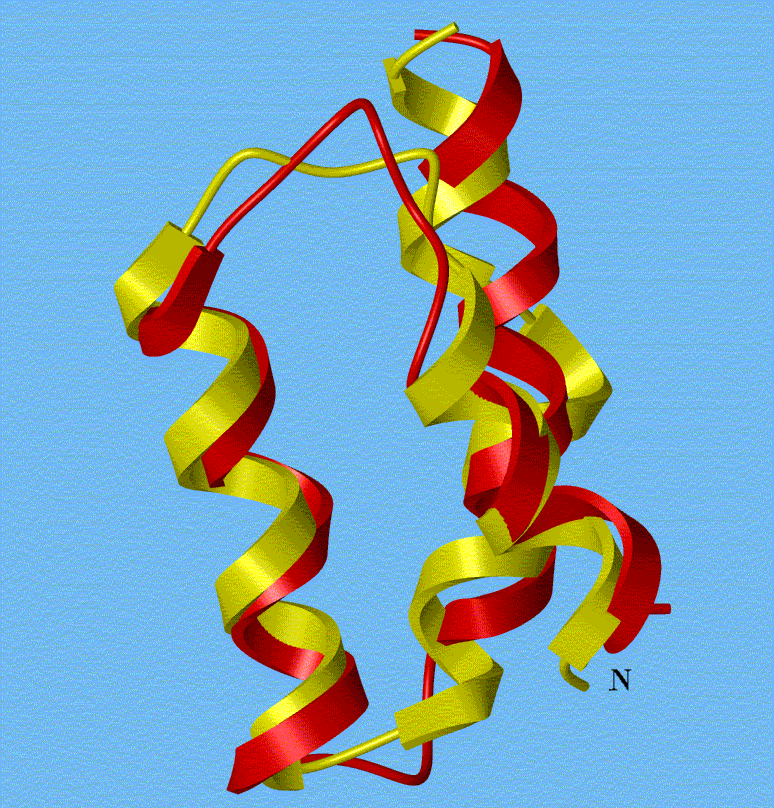 “Atomically
detailed folding simulation of the B domain of staphylococcal protein A from
random structures.”
J.A. Vila, D.R. Ripoll, and H.A. Scheraga. (2003). Proc. Natl
Acad. Sci. USA. 100, 14812-14816.[pdf]
“Atomically
detailed folding simulation of the B domain of staphylococcal protein A from
random structures.”
J.A. Vila, D.R. Ripoll, and H.A. Scheraga. (2003). Proc. Natl
Acad. Sci. USA. 100, 14812-14816.[pdf]
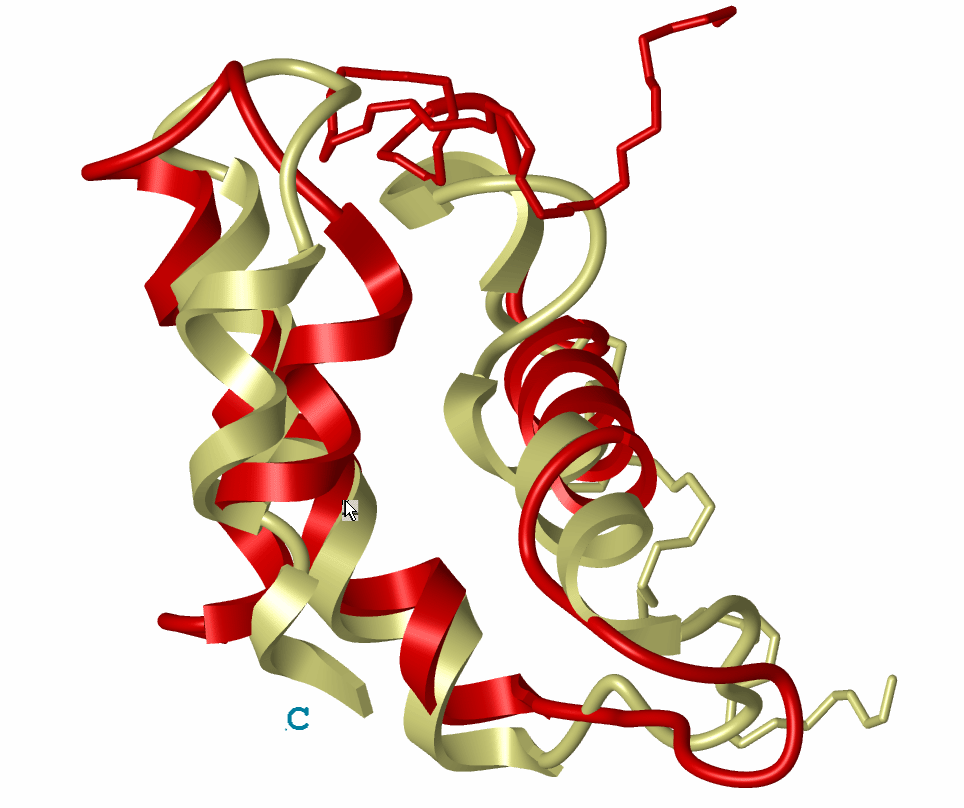 A. Liwo, J. Lee, D.R. Ripoll, J. Pillardy, and H.A. Scheraga. (1999). “Protein Structure
Prediction by Global Optimization of a Potential Energy Function”.
Proc. Natl. Acad. Sci. USA, 96, 5482-5485.
[pdf]
A. Liwo, J. Lee, D.R. Ripoll, J. Pillardy, and H.A. Scheraga. (1999). “Protein Structure
Prediction by Global Optimization of a Potential Energy Function”.
Proc. Natl. Acad. Sci. USA, 96, 5482-5485.
[pdf]
J. Lee, A. Liwo, D.R. Ripoll, J.Pillardy, J.A. Saunders, K.D. Gibson, and H.A. Scheraga.. (2000). “Hierarchical energy-based approach to protein-structure prediction; blind-test evaluation with CASP3 targets”. Int. J. Quant. Chem. 77, 90-117. [pdf]
``A Molecular Switch for Biological Logic Gates: Conformational Studies.'' G. Ashkenazi, D. R. Ripoll, N. Lotan, H. A. Scheraga. (1997). Biosensors & Bioelectronics 12, 85-95. [pdf]
 J.A. Vila, D.R. Ripoll and H.A. Scheraga. (2001). “Influence of Lysine
Content and pH on the Stability of Alanine-Based Co-polypeptides”.
Biopolymers, 58, 235-246.
[pdf]
J.A. Vila, D.R. Ripoll and H.A. Scheraga. (2001). “Influence of Lysine
Content and pH on the Stability of Alanine-Based Co-polypeptides”.
Biopolymers, 58, 235-246.
[pdf]
J.A. Vila, D.R. Ripoll and H. A. Scheraga. (2000). “Physical reasons for the unusual α-helix stabilization afforded by charged or neutral polar residues in alanine-rich peptides”. Proc. Natl. Acad. Sci. USA. 97, 13075-13079. [pdf]
Coupling between Folding and Ionization Equilibrium. Effects of pH on the Conformational Preference of Oligopeptides. D. Ripoll, Y. Vorabjev, A. Liwo, H. Scheraga and J. Vila. (1996). J. Mol. Biol. 264, 770-783. [pdf]
To see another figure showing the electrostatic field and surface potential generated by one of the lowest energy conformation click here.
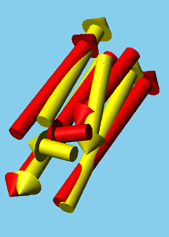
M. Nanias, M. Chinchio, J. Pillardy, D.R. Ripoll, and H. A. Scheraga. (2003). “Packing helices in proteins by global optimization of a potential energy function.” Proc. Natl. Acad. Sci. USA, 100, 1706-1710. [pdf]