ELECTROSTATICS OF THE RECOMBINANT HUMAN H-CHAIN FERRITIN
 Ferritin, the iron storage protein, is central to mechanisms of iron
homeostasis in biological systems.
In addition, the protein has attracted interest as a
spatially constrained reaction environment for the synthesis of nanophase
inorganic materials.
Mammalian ferritin is composed of 24 polypeptide subunits
forming a nearly spherical shell around a central cavity,
roughly 80 in diameter, in which a hydrated iron(III) oxide
(ferrihydrite) is mineralized.
Ferritin, the iron storage protein, is central to mechanisms of iron
homeostasis in biological systems.
In addition, the protein has attracted interest as a
spatially constrained reaction environment for the synthesis of nanophase
inorganic materials.
Mammalian ferritin is composed of 24 polypeptide subunits
forming a nearly spherical shell around a central cavity,
roughly 80 in diameter, in which a hydrated iron(III) oxide
(ferrihydrite) is mineralized.
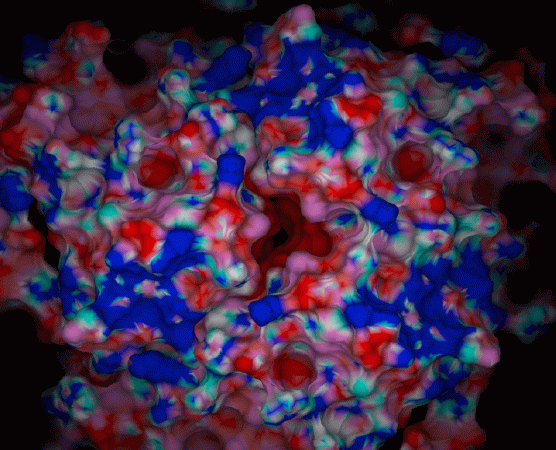
The proposed mechanism for the mineralization reaction involves the protein catalyzed oxidation of Fe(II) by molecular oxygen, and the site directed nucleation of the iron oxide mineral inside the protein cavity. Active sites for the ferroxidase center (His 65, Glu 62, Glu 27) and nucleation center (Glu 61, Glu 64, Glu 67) have been identified.
Results theoretical calculations were undertaken to investigate the influence of the electrostatic field generated by the protein on this unique inorganic mineralization reaction. The presence of large electrostatic field on the outer surface of the protein illustrates unambiguously the pathway of Fe(II) entering the protein. In addition, some patterns coming out from these electrostatic calculations have led us to identify a group of conserved residues that could be implicated in the mechanism for proton translocation across the protein shell. The presence of a large electrostatic field through the 4-fold channel, previously thought to be apolar, suggests the possibility that this channel may function as a pathway for the removal of protons from within the protein cavity. This previously un-investigated aspect of the reaction has implications for our understanding of the mineral produced and the kinetics of this protein catalyzed mineralization reaction.
Publication:
T. Douglas and D.R. Ripoll. (1998). “Calculated electrostatic Gradients in Recombinant Human H-Chain Ferritin”. Protein Sci., 7, 1083-1091. [pdf]