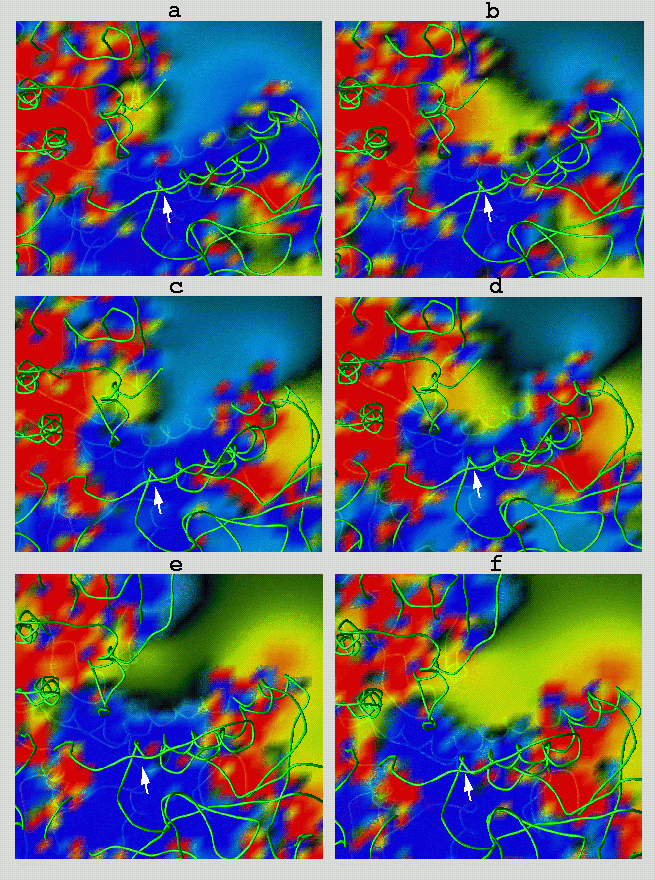
Two structurally related disulfide metabolites, the well-known glutathione (GSSG)
and its analog trypanothione (TS2), which is found in trypanosomatid parasites,
have different net charges of -2 (GSSG) and +1 (TS2). Three structurally known
enzymes which reduce these substrates, human glutathione reductase (hGR), Escherichia
coli glutathione reductase (ecGR) and Crithidia fasciculata trypanothione reductase
(cfTR) show differential specificity (kcat/Km) for GSSG versus TS2 of 104:1, 102:1
and 10-8:1, respectively. In the figure, the electrostatic isopotential surfaces
in the active sites of these enzymes are color coded according to the value of
the potential: dark blue 3< f; light blue 0< f< 3; green -1.5< f< 0; yellow
-3
This clearly shows an electrostatic preference of hGR for GSSG over TS2. The electrostatic
gradient of hGR (not shown) will attract GSSG toward the active site. It has been shown
experimentally that hGR can be engineered into a trypanothione reductase (TR) [1], thus
changing the relative selectivity for GSSG over TS2. Panel b) displays the isopotentials for
hGR(A34E:R37W), respectively. The change in the isopotential surfaces after the double
mutation illustrates the electrostatic complementarity between the mutated enzyme and TS2
and is consistent with the increased selectivity (103) of this mutated enzyme toward TS2.
E. Coli GR , on the other hand, shows less selectivity for GSSG over TS2 (2x102). By
mutating three residues, A18E:N21W:R22N, E. Coli GR specificity for TS2 was increased [2].
Panels c) and d) display the corresponding isopotentials calculated for E. Coli GR(wild type)
and E.Coli (A18E:N21W:R22N). The appearence of additional attractive isopotentials for TS2
is apparent. TR shows an exquisite selectivity for TS2 over GSSG by a factor of 108. A
triple mutant of TR, TR(E18A:W21R:A343R) [3], acquired GR activity, changing the relative
selectivity for TS2 over GSSG by 107. Panels e) and f) show the corresponding electrostatic
potentials for both TR(E18A:W21R:A343R) and TR, respectively.
The above examples illustrate the electrostatic complementarity between GR and GSSG
and TR and TSST, their natural substrates. Furthermore, electrostatic isopotentials follow
the changes in substrate selectivity upon specific mutations.
References